Min Liu, Fen-Xiang Li, Chun-Yuan Li, Xiao-Cong Li, Long-Fei Chen, Kun Wu, Pei-Liang Yang, Zhi-Fa Lai, Ting-kai Liu, William J. Sullivan Jr., Liwang Cui, Xiao-Guang Chen*
ABSTRACT
Background: Protein arginine methylation is a prevalent post-translational modifcation. The protein arginine methyltransferase family (PRMT) is involved in many cellular processes in eukaryotes, including transcriptional regulation, epigenetic regulation, RNA metabolism, and DNA damage repair. Toxoplasma gondii, an opportunistic protozoan parasite, encodes fve conserved PRMTs. PRMT5 is thought to be responsible for substantial PRMT activity in T. gondii;
however, it has not yet been characterized.
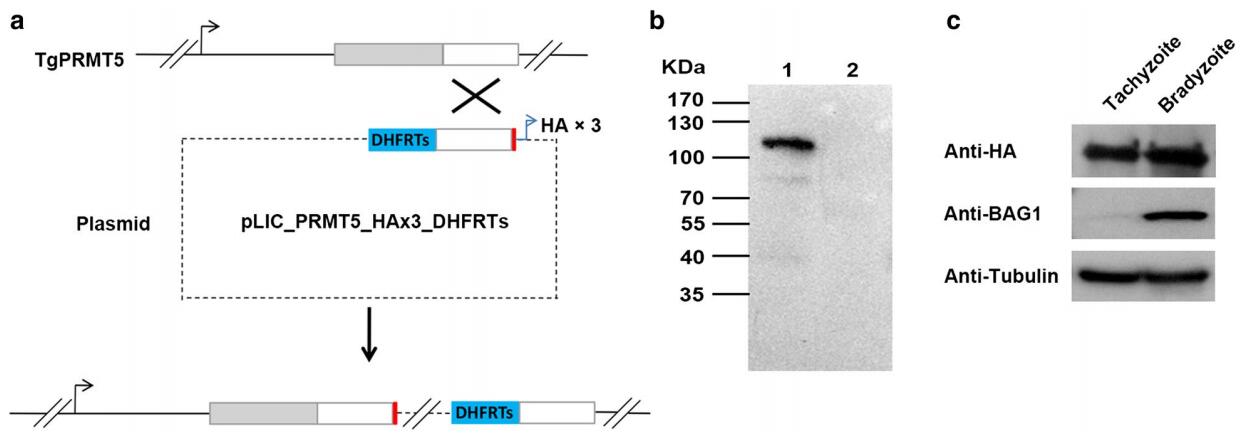
Methods: We tagged the 3′ end of the endogenous TgPRMT5 genomic locus with sequence encoding a 3X hemagglutinin (HA) epitope. IFA and WB were performed to check the expression and subcellular localization of TgPRMT5 in tachyzoites and bradyzoites. In vitro methylation assays were performed to determine whether endogenous TgPRMT5 has arginine methyltransferase activity.
Results: IFA and WB results showed that T. gondii PRMT5 (TgPRMT5) was localized in the cytoplasm in the tachyzoite stage; however, it shifts largely to the nuclear compartment in the bradyzoite stage. The in vitro methylation showed that TgPRMT5 has authentic type II PRMT activity and forms monomethylarginines and symmetric dimethylarginines.
Conclusions: We determined the expression and cellular localization of TgPRMT5 in tachyzoites and bradyzoites and confrmed its type II PRMT activity. We demonstrated the major changes in expression and cellular localization of TgPRMT5 during the tachyzoite and bradyzoite stages in T. gondii. Our fndings suggest that TgPRMT5 protein may be involved in tachyzoite-bradyzoite transformation.
Parasites Vectors. 2019.
DOI : https://doi.org/10.1186/s13071-019-3464-1