Tong Liu, Wen-Qiang Yang, Yu-Gu Xie, Pei-Wen Liu, Li-Hua Xie, Feng Lin, Chen-Ying Li, Jin-Bao Gu, Kun Wu, Gui-Yun Yan* and Xiao-Guang Chen*
ABSTRACT
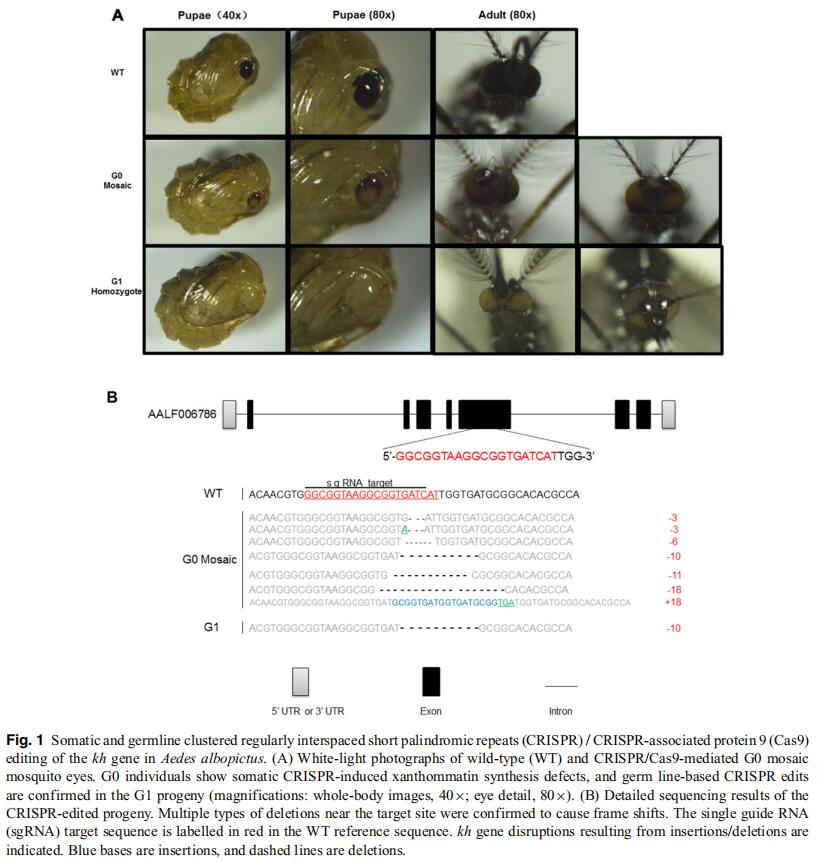
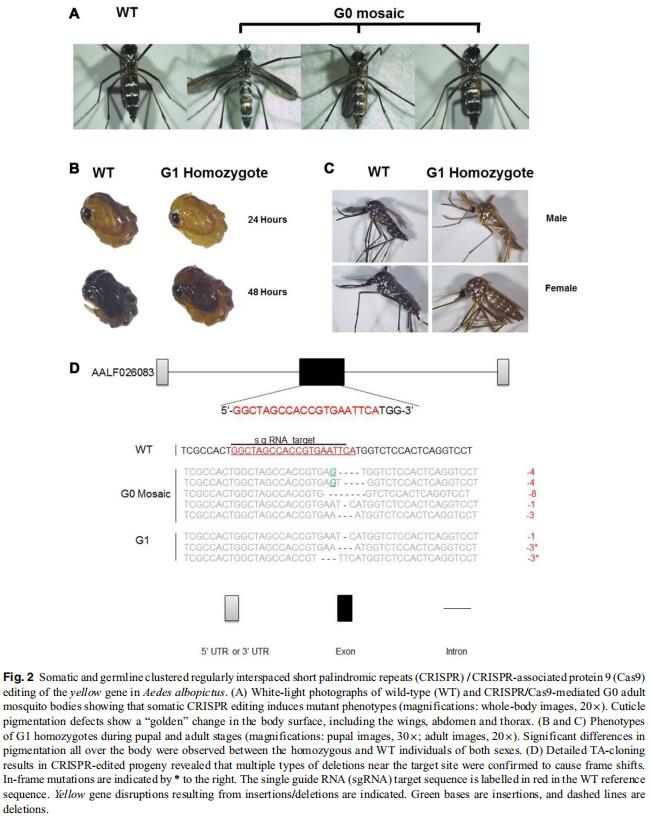
Aedes (Stegomyia) albopictus, also known as the Asian tiger mosquito, is a mosquito which originated in Asia. In recent years, it has become increasingly rampant throughout the world. This mosquito can transmit several arboviruses, including dengue, Zika and chikungunya viruses, and is considered a public health threat. Despite the urgent need of genome engineering to analyze specific gene functions, progress in genetical manipulation of Ae. albopictus has been slow due to a lack of efficient methods and genetic markers. In the present study, we established targeted disruptions in two genes, kynurenine hydroxylase (kh) and dopachrome conversion enzyme (yellow), to analyze the feasibility of generating visible phenotypes with genome editing by the clustered regularly interspaced short palindromic repeats (CRISPR) / CRISPR-associated protein 9 (Cas9) system in Ae. albopictus. Following Cas9 single guide RNA ribonucleoprotein injection into the posterior end of pre-blastoderm embryos, 30%–50% of fertile survivors produced alleles that failed to complement existing kh and yellow mutations. Complete eye and body pigmentation defects were readily observed in G1 pupae and adults, indicating successful generation of highly heritable mutations. We conclude that the CRISPR/Cas9-mediated gene editing system can be used in Ae. albopictus and that it can be adopted as an efficient tool for genome-scale analysis and biological study.
DOI : https://doi.org/10.1111/1744-7917.12645